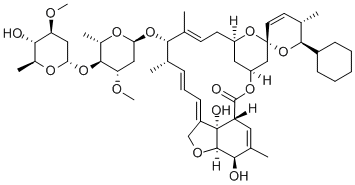
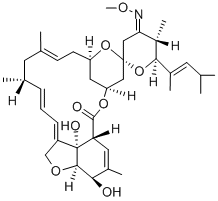
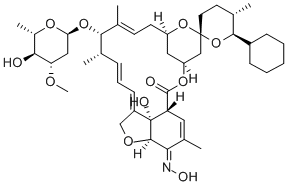
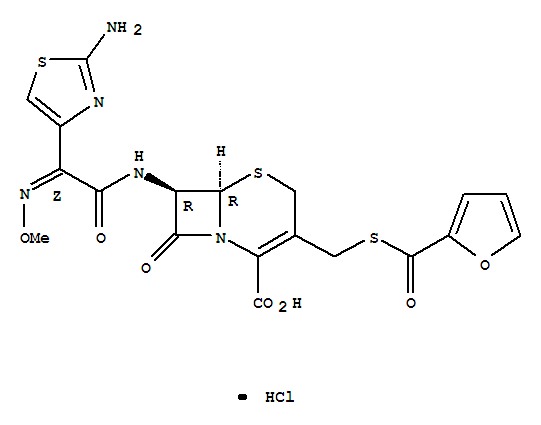
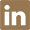Ceftiofur Hydrochloride Manufacturer Supply High Quality 103980-44-5 with Good Price
- Molecular Formula:C19H18ClN5O7S3
- Molecular Weight:560.02
- Appearance/Colour:Off-white solid
- Vapor Pressure:5.14E-28mmHg at 25°C
- Melting Point:>190°C (dec.)
- Boiling Point:814.1 °C at 760 mmHg
- Flash Point:446.1 °C
- PSA:256.26000
- LogP:2.69300
Ceftiofur hydrochloride(Cas 103980-44-5) Usage
| Uses | Ceftiofur hydrochloride is a broad-spectrum, third-generation cephalosporin antibiotic used primarily in veterinary medicine to treat various bacterial infections. It's effective against a wide range of both Gram-positive and Gram-negative bacteria, including strains that produce β-lactamase enzymes which typically render penicillin ineffective. |
|
Veterinary Drugs and Treatments |
In swine, ceftiofur HCl injection is labeled for the treatment and control of swine bacterial respiratory disease (swine bacterial pneumonia) associated with Actinobacillus (Haemophilus) pleuropneumoniae, Pasteurella multocida, Salmonella choleraesuis and Streptococcus suis. In cattle, ceftiofur HCl is labeled for the treatment of the following bacterial diseases: Bovine respiratory disease (BRD, shipping fever, pneumonia) associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni; Acute bovine interdigital necrobacillosis (foot rot, pododermatitis) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus; and acute metritis (0 – 14 days post-partum) associated with bacterial organisms susceptible to ceftiofur. The intramammary syringe for dry dairy cattle (Spectramast DC?) is labeled for the treatment of subclinical mastitis in dairy cattle at the time of dry off associated with Staphylococcus aureus, Streptococcus dysgalactiae, and Streptococcus uberis. The intramammary syringe for lactating dairy cattle (Spectramast LC?) is labeled for the treatment of clinical mastitis in lactating dairy cattle associated with coagulase-negative staphylococci, Streptococcus dysgalactiae, and Escherichia coli. |
|
Brand Name(s) in US |
Jiangsu Willing Bio-Tech Co., Ltd is a new biotech factory which was builted in 2012 by Jiangsu Lingyun Pharmaceutical Co., Ltd in Huai'an, Jiangsu Province. It covers an area of 135, 00 square meters and is constructed according to GMP requirements. The line covers fermentation, extraction, synthesis, refining and packing. Willing Bio-Tech has obtained Chinese GMP certificate and passed USA FDA inspection in 2017. We have passed the veterinary GMP inspection under the updated version of 2022. |
InChI:InChI=1/C19H17N5O7S3.ClH/c1-30-23-11(9-7-34-19(20)21-9)14(25)22-12-15(26)24-13(17(27)28)8(5-32-16(12)24)6-33-18(29)10-3-2-4-31-10;/h2-4,7,12,16H,5-6H2,1H3,(H2,20,21)(H,22,25)(H,27,28);1H/b23-11+;