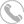Products
| Product Name | Cas No | Specification | Inquiry |
|---|---|---|---|
| Zilpaterol hydrochloride | 119520-06-8 | 99% | Inquiry |
| Toltrazuril | 69004-03-1 | IH HOUSE/ CVP/ASMF | Inquiry |
| Tulathromycin | 217500-96-4 | IH HOUSE/ VMF/ASMF | Inquiry |
| Doramectin | 117704-25-3 | IH HOUSE/ VMF/ASMF | Inquiry |
| Moxidectin | 113507-06-5 | EP/USP/ CEP/VMF | Inquiry |
| Selamectin | 220119-17-5 | EP/USP/ CEP/VMF | Inquiry |
| Oclacitinib Maleate | 1208319-27-0 | R&D | Inquiry |
| Imidacloprid | 138261-41-3 | VMF/CEP | Inquiry |
| Milbemycin Oxime | 129496-10-2 | EP/USP | Inquiry |
| Spinosad | 131929-60-7 | R&D | Inquiry |
| Decoquinate | 18507-89-6 | USP/ASMF | Inquiry |
| Fipronil | 120068-37-3 | IN HOUSE/CVP | Inquiry |
| Ponazuril | 69004-04-2 | IN HOUSE | Inquiry |
| Fluralaner | 864731-61-3 | IN HOUSE/ VMF/ASMF | Inquiry |
| Tiamulin Fumarate | 55297-96-6 | USP/CVP | Inquiry |
| 1-Methylhexahydroazepin-4-one Hydrochloride | 19869-42-2 | ≥98.0% | Inquiry |
| Isocyanate distilled | 106310-19-4 | 99% | Inquiry |
| Adipic acid dihydrazide | 1071-93-8 | ≥99.3% | Inquiry |
| Valnemulin Hydrochloride | 133868-46-9 | IH HOUSE/CVP | Inquiry |
| Cefquinome Sulfate | 118443-89-3 | IH HOUSE | Inquiry |
Product Name: Zilpaterol hydrochloride
Cas No: 119520-06-8
Molecular Formula: C14H19N3O2.HCl
Specification: 99%
InquiryProduct Name: Toltrazuril
Cas No: 69004-03-1
Molecular Formula: C18H14F3N3O4S
Specification: IH HOUSE/ CVP/ASMF
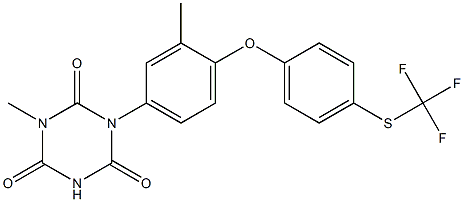
InquiryProduct Name: Tulathromycin
Cas No: 217500-96-4
Molecular Formula: C41H79N3O12
Specification: IH HOUSE/ VMF/ASMF
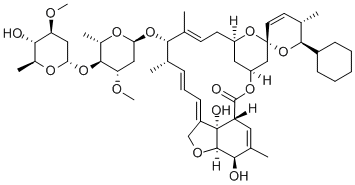
InquiryProduct Name: Doramectin
Cas No: 117704-25-3
Molecular Formula: C50H74O14
Specification: IH HOUSE/ VMF/ASMF
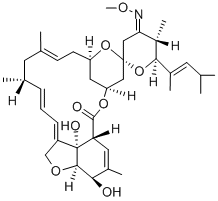
InquiryProduct Name: Moxidectin
Cas No: 113507-06-5
Molecular Formula: C37H53NO8
Specification: EP/USP/ CEP/VMF
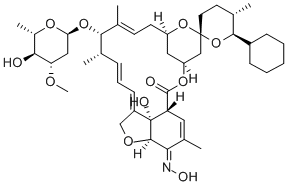
InquiryProduct Name: Selamectin
Cas No: 220119-17-5
Molecular Formula: C43H63NO11
Specification: EP/USP/ CEP/VMF
InquiryProduct Name: Oclacitinib Maleate
Cas No: 1208319-27-0
Molecular Formula:
Specification: R&D
InquiryProduct Name: Imidacloprid
Cas No: 138261-41-3
Molecular Formula: C9H10ClN5O2
Specification: VMF/CEP
InquiryProduct Name: Milbemycin Oxime
Cas No: 129496-10-2
Molecular Formula: C63H88N2O14
Specification: EP/USP
InquiryProduct Name: Decoquinate
Cas No: 18507-89-6
Molecular Formula: C24H35NO5
Specification: USP/ASMF
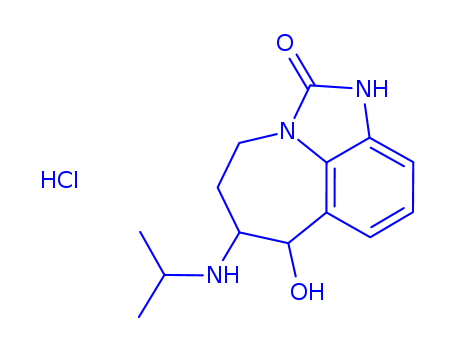
InquiryProduct Name: Fipronil
Cas No: 120068-37-3
Molecular Formula: C12H4Cl2F6N4OS
Specification: IN HOUSE/CVP
InquiryProduct Name: Ponazuril
Cas No: 69004-04-2
Molecular Formula: C18H14F3N3O6S
Specification: IN HOUSE
InquiryProduct Name: Fluralaner
Cas No: 864731-61-3
Molecular Formula: C22H17Cl2F6N3O3
Specification: IN HOUSE/ VMF/ASMF
InquiryProduct Name: Tiamulin Fumarate
Cas No: 55297-96-6
Molecular Formula: C32H51NO8S
Specification: USP/CVP
InquiryProduct Name: 1-Methylhexahydroazepin-4-one Hydrochloride
Cas No: 19869-42-2
Molecular Formula: C7H13NO.HCl
Specification: ≥98.0%
InquiryProduct Name: Isocyanate distilled
Cas No: 106310-19-4
Molecular Formula: C15H10F3NO2S
Specification: 99%
InquiryProduct Name: Adipic acid dihydrazide
Cas No: 1071-93-8
Molecular Formula: C6H14N4O2
Specification: ≥99.3%
InquiryProduct Name: Valnemulin Hydrochloride
Cas No: 133868-46-9
Molecular Formula: C31H53ClN2O5S
Specification: IH HOUSE/CVP
InquiryProduct Name: Cefquinome Sulfate
Cas No: 118443-89-3
Molecular Formula: C23H26N6O9S3
Specification: IH HOUSE
Inquiry